Medical device manufacturers are by nature, rather aloof when it comes to the public.
There is little to be gained, and much to be lost.
Typically whenever a medical device manufacturer makes the news, it is usually for something bad – a recall, an adverse patient event, a regulatory sanction.
Less is more, particularly when it comes to direct patient engagement, a belief ubiquitous across the industry.
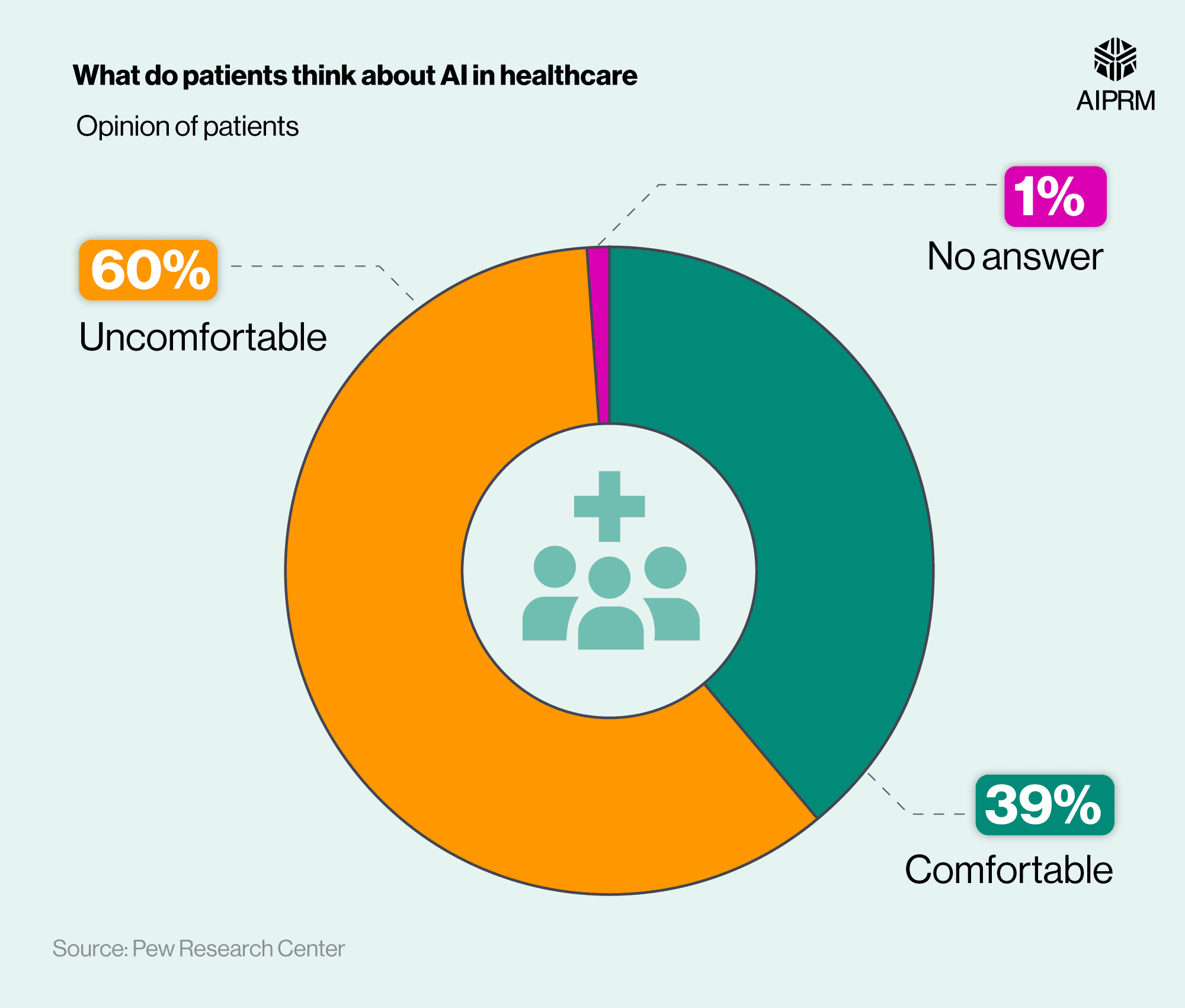
A belief primed to change in the post-pandemic world of healthcare. A recent study by McKinsey & Co. has found that an overwhelming percentage of both healthcare providers and patients desire a change in overall engagement with medical devices. A change we are starting to see.
Whereas before the engagement was defined by the healthcare policy, new models of engagement center on aspects of the patient journey not previously associated with medical device companies.
A supplier of in vitro diagnostics conducted ethnographic studies to address unmet needs of its diverse patient population, needs created by healthcare disparities overlooked in pre-pandemic healthcare.
The pandemic has changed the perception of healthcare, and the expectations for good clinical care. It naturally follows that the interactions change as well. Medical devices are in a prime position to capitalize on this shift by exploring novel value creating opportunities – primarily through new forms of patient engagement.
Early, successful attempts at capturing these opportunities emphasize the patient journey. Subsequent attempts should expand upon the patient journey, and transform medical devices from a product to be sold into an advocate for the patient.
A device as intimate to the patient as a smart phone, and as integral to patient care as the providers themselves.
To make such a shift, medical devices should adjust how they view themselves first and foremost. Steve Jobs revolutionized the smart phone industry by emphasizing the intimate relationship between a person and their phone, seeing technology in terms of emotions rather than of hardware. He did not invent any new technology; he simply made existing technology intimate.
Medical device manufacturers must see themselves as intimate companions in the patient journey, walking alongside the patient, as an advocate, supporting as much as treating the patient.
Instead of devices monitoring blood sugar, we need devices that encourage patients to comply with a diabetic diet while monitoring blood sugar. Instead of devices that monitor urine output, we need devices that support patients during moments of acute kidney injuries.
The perception of the treatment matters as much as the treatment itself. And the engagement with the patient matters as much as the effectiveness of the treatment.
Medical device manufacturers successfully able to transform their devices into patient advocates provide key stakeholders – whether they are hospitals, healthcare insurance companies, or care providers – a point of engagement through which they – device manufacturers and stakeholders – can improve patient compliance together.
Compliance largely directed through changes in patient perception prompting behavioral changes in the patient.
Changes that optimize the treatment provided.
Changes that have always been the most important aspect of healthcare.
Changes we were abruptly reminded of during the pandemic.