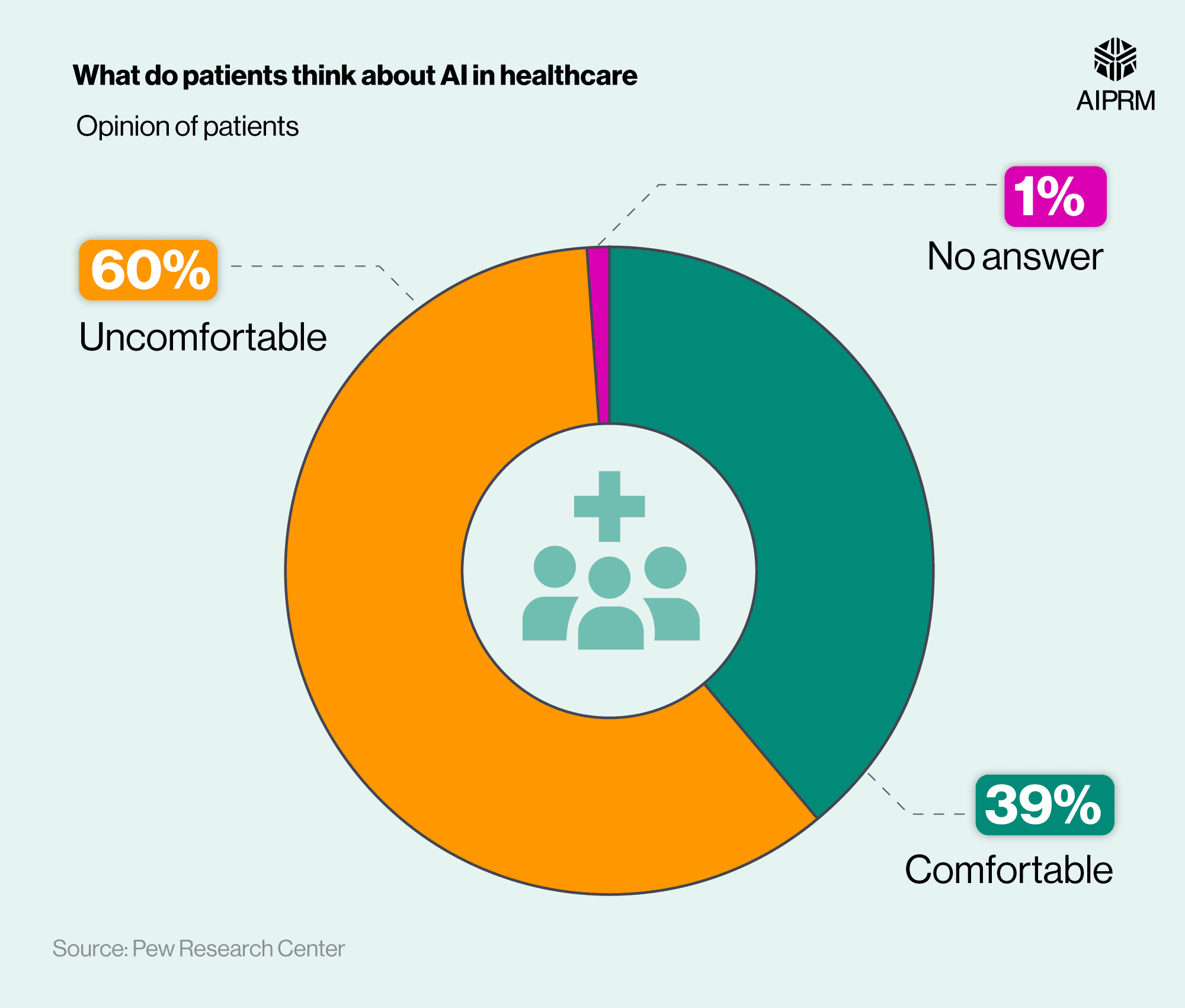
Therefore, it is crucial to examine the research surrounding these medications with a critical eye to uncover any potential bias that may impact the validity of the findings. While there are no studies to suggest malfeasance or outright misrepresentation, we encourage readers to keep a critical eye on the news coming out about these medications. And keep a particularly keen focus on the studies underlying the news headlines.
The importance of unbiased clinical studies in healthcare
Unbiased clinical studies play a critical role in shaping evidence-based healthcare practices. By scrutinizing research methodologies and data interpretation, healthcare professionals can make informed decisions that drive patient care advancements. When bias creeps into clinical studies, it can jeopardize the integrity of the findings and ultimately impact patient outcomes. In the realm of GLP-1 medication research, unbiased studies are paramount to ensure that patients receive safe and effective treatments based on accurate evidence. Moving forward, healthcare practitioners must prioritize transparency, objectivity, and rigor in clinical research to uphold the highest standards of patient care and medical advancement.
Understanding the potential for bias in GLP-1 medication research
For GLP-1 medication research, when studying new clinical applications, it is crucial to acknowledge the potential for bias that may influence study outcomes. Various forms of bias, such as selection bias, publication bias, and funding bias, can skew research findings and compromise the integrity of clinical studies. Researchers and healthcare professionals must remain vigilant in identifying and mitigating these biases to ensure that patients receive unbiased and reliable information on the efficacy and safety of GLP-1 medications. By recognizing and addressing bias in research, we can uphold the credibility of clinical studies and ultimately deliver evidence-based healthcare that prioritizes patient well-being and optimal treatment outcomes.
Addressing common biases in clinical studies
To combat biases in GLP-1 medication research, researchers must be open about their research methodologies and practices. One key strategy is to prioritize transparency by disclosing all study protocols, including data collection and analysis methods. Utilizing blinding techniques can help minimize bias by preventing researchers and participants from being influenced by preconceived notions. Additionally, conducting independent peer reviews and replicating studies can validate findings and ensure their robustness. By actively addressing biases and promoting transparency in clinical studies, we can enhance the reliability and trustworthiness of research outcomes, ultimately leading to more informed healthcare decisions for patients.
Strategies for minimizing bias in research
In addition to transparency and blinding techniques, researchers can implement several other strategies to minimize bias in GLP-1 medication studies. Randomizing participant assignment, ensuring adequate sample sizes, and controlling for confounding variables are essential steps to enhance the validity of study results. Emphasizing adherence to established research protocols and ethical standards can further mitigate biases. Utilizing diverse study populations and incorporating interdisciplinary perspectives can also provide valuable insights and minimize the risk of narrow-minded biases. Employing these strategies in conjunction with transparent practices can foster credibility and reliability in clinical research, ultimately advancing the efficacy and safety of GLP-1 medications.
The impact of biased clinical studies on patient care
Biased clinical studies can have far-reaching implications on patient care. Misleading study results influenced by bias can lead to inappropriate treatment decisions, compromising patient safety and well-being. Patients may be subjected to ineffective or potentially harmful medications if research findings are not rigorously unbiased. Moreover, biased studies can disrupt medical guidelines and hinder healthcare professionals’ ability to make evidence-based decisions. Ultimately, patients bear the brunt of skewed research outcomes, facing challenges in accessing optimal care and personalized treatment plans. It is imperative for the scientific community to prioritize unbiased research practices to safeguard patient health and improve clinical outcomes.
Conclusion: the need for transparency and integrity in medical research
The prevalence of bias in clinical studies, which includes research on GLP-1 medications, underscores the critical importance of transparency and integrity in medical research. It is true that nothing of concern has been found to date. However, to ensure the validity and reliability of study results, researchers must uphold rigorous standards of impartiality and objectivity throughout the research process. Transparency in reporting methodologies, results, and potential conflicts of interest is essential to fostering trust in the scientific community and promoting evidence-based healthcare practices. By prioritizing unbiased research practices and adhering to ethical guidelines, we can enhance patient care, advance medical knowledge, and ultimately, improve clinical outcomes for all. Let us commit to maintaining the highest standards of integrity in medical research to uphold the well-being of patients and the integrity of our healthcare system.