The world’s largest democracy is about to face a once in a life time challenge, begging the question – can India effectively administer the COVID-19 vaccine to its high risk population?
India is set to begin its roll-out in the coming days, but if the issues and set backs other countries have faced are any indication, then India may have its hands full.
India has confirmed over 10 million COVID-19 cases, second in the world to the United States, though around 150,000 people have died from the virus in India, compared to 375,000 deaths in the United States.
India plans to inoculate 300 million people in the first phase of the vaccination program, which includes healthcare and front-line workers, police, and military troops, as well as those with comorbidities who are above the age of 50.
India will lean heavily on the vaccine produced by Serum Institute of India, the world’s largest vaccine manufacturing company, with a stellar record of producing vaccines for tropical countries.
Serum Institute of India’s vaccine does not require the ultra-cold storage facilities that others do. Instead, it can be stored in traditional refrigerators, reducing the cost of storage significantly for a country with a tropical climate.
India, much like the United States, has multiple vaccines available, both domestically and internationally developed. It has a well-defined prioritization protocol that it promises will not be compromised. But unlike the United States, it does not have the storage requirements that the United States has, and faces significantly less logistical challenges than the United States.
But the vaccine India will use has proven less effective than the vaccines in the United States.
Pfizer and Moderna have touted vaccine efficacies well into the 90th percentile, higher than any of the vaccines available in India. But because Pfizer and Moderna require extremely cold storage temperatures and have a relatively short shelf-life, the United State’s government has had to manage a tricky logistical balance of storing and distributing the vaccines at capacities intended to predict the anticipated demand for the vaccine per locale.
Needless to say, that has not always worked out. And a large reason why many vaccines have been delayed stems from the disconnect in anticipated demand and projected supply, with many vaccines sitting in siloes ready to be deployed without a set timeline for when they will be administered.
India, in contrast, has numerous vaccine manufacturers located across major distribution hubs ready to produce the vaccine in a short time. Manufacturing vaccines has never been an issue in India, and India’s strong international presence as an exporter of vaccines has established a foundation for a seamless distribution model domestically.
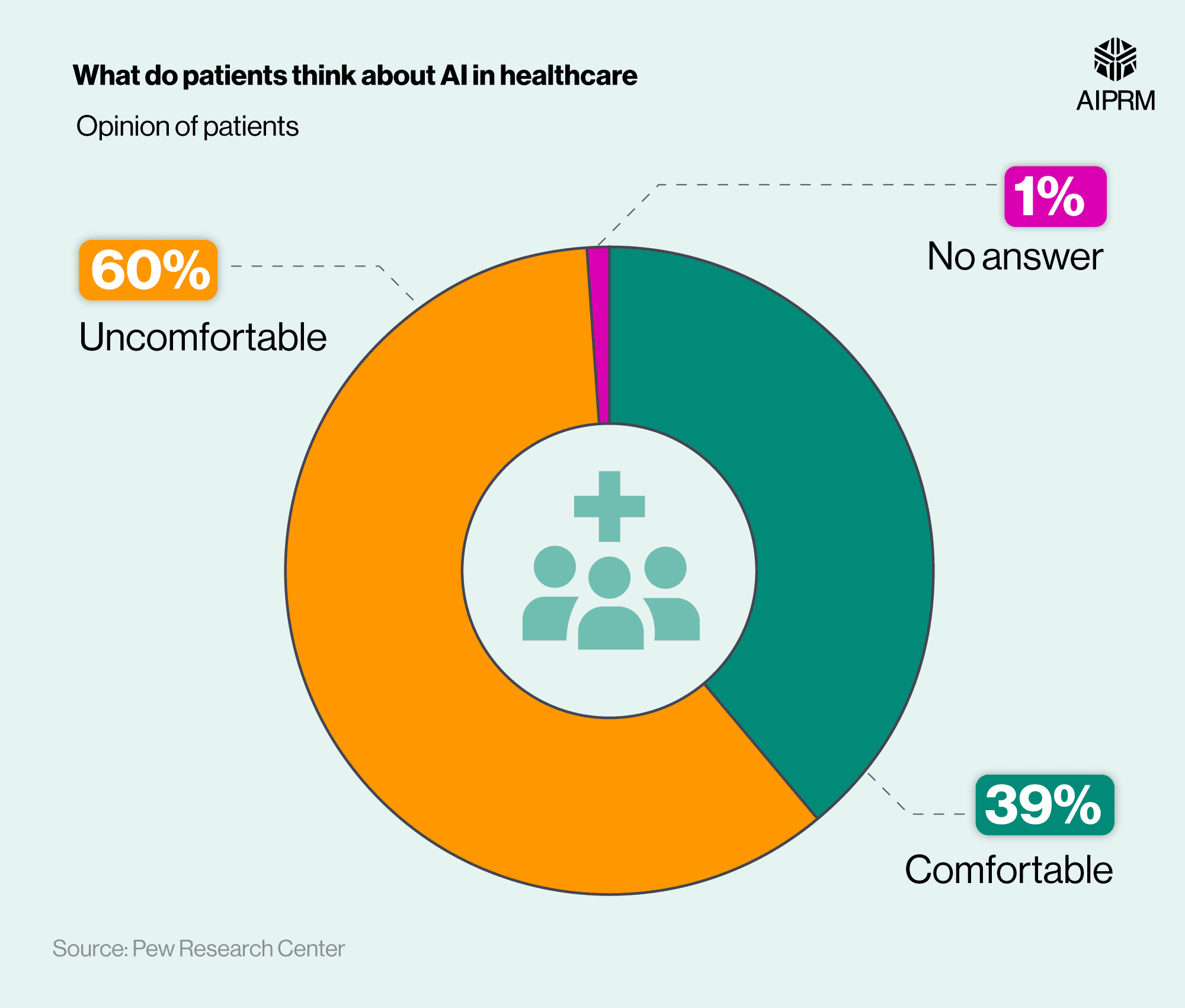
The other, perhaps equally important reason India will be successful is the exceedingly high confidence the Indian public has in taking the vaccine. An international survey found that Indians are the most keen in the world on getting vaccinated whenever a vaccine becomes available to them, even as people in 10 out of 15 countries show a growing reluctance about getting vaccinated.
The survey found that 87% of Indians who responded are intent on receiving the vaccine, despite 34% being worried about side effects, and 16% being concerned about the fast-moving trials. Unlike other countries, however, these concerns have not deterred the willingness to get vaccinated. A unique predisposition that may bode well for a successful vaccination campaign.
In the United States we find unexpected, widespread hesitancy to receive the vaccine, even among healthcare and front-line workers. That sentiment does not exist in India. The counter-culture sense of distrust in science seems to be absent in India, and may result in a greater percentage of the population willing to take the vaccine.
Additionally, and perhaps most uniquely, 93% of the respondents surveyed believe the Indian government, particularly their prime minister, Narendra Modi, is doing a good job handling the COVID-19 response. That unprecedented level of trust in a country’s leader is typically reserved for times of war, and is nearly unheard of among other countries.
Likely the strong response and faith in the Indian government correlates to a strong willingness to take the vaccine. Indicating that demand for the vaccine is likely to remain steadfast among the Indian population throughout the vaccine administration process.
There is much to be optimistic about India’s vaccine roll-out. And there is much we in the United States can learn.
India early on made a clear decision to develop a vaccine that had less stringent storage requirements and more adept for distribution, though the efficacy has proven to be lower than Pfizer and Moderna. The logistical difficulties we face could have been overcome by a vaccine that is easier to transport. And realistically, whether a vaccine is 90% effective or 80% effective matters less than whether the vaccine gets into a patient’s arm.
India has a robust distribution network developed over many years by its vaccine manufacturing industry. And the COVID-19 vaccine will be distributed along this network as it reaches the major cities and rural villages of India. In contrast, the United States developed its own distribution networks through the military via Operation Warp Speed. As the federal government presumed that the military would be better at distributing the vaccine.
But why create a network from scratch as opposed to utilizing existing distribution networks? A seemingly obvious decision the United States got wrong.
The United States could have relied on medical distributors to store and distribute the vaccine. Instead, it created ad hoc partnerships throughout the country depending on available sub-thermal storage capacity.
India’s logistical advantage over the United States will serve India well early in the vaccine administration process. There will be fewer incidences of vaccines spoiling or vaccines not reaching its intended location in time.
But India’s sustained success is will come from the consistent and overwhelmingly high demand for the vaccine. It is easy to know how many vaccines to distribute in a given town when you can assume nearly every person will take the vaccine. It is more difficult when the demand waxes and wanes based upon recent tweets or political rhetoric.
The difficulties the United States have faced with counter-cultural and anti-science movements manifested most prominently when the vaccine first became available.
Demand dropped as many Americans were reluctant to be the first vaccinated. And only rose after millions upon millions of Americans received the vaccine. In India there is a low chance of any initial hesitancy, and it likely that the willingness to take the vaccine will remain high throughout the campaign.
Giving India an enormous opportunity to prove itself on the world stage. And giving the United States and other countries an opportunity to learn how to emulate a nationwide distribution model for vaccines.