The State AG’s Nationwide Opioid Settlement Patient Harm
About a year ago, I was first alerted to the negative impact of the State Attorneys General’s nationwide opioid settlement with the three major distributors/wholesalers of controlled substances to retail pharmacies, Cardinal Heath, McKesson, and AmerisourceBergen. Patricia (Pat) Irving, an RN who retired as National Leader for Risk and Patient Safety for Kaiser Permanente (KP) was personally experiencing the direct and very negative impact of this settlement, specifically it’s “Injunctive Relief”. She thankfully chose to “sound the alarm” about this emerging harm to the entire chronic pain community (CPP).
The engineer in me compelled the twice reading of all 54 pages of the injunction, which is Exhibit P of the 571-page settlement! This unpleasant experience convinced me Pat was speaking the frightening truth. She and I started communicating and soon became good friends. We have joined forces in attempting to grow awareness of this latest assault on the entire CPP community, patients, physicians, and pharmacists alike.
In this writing we wish to share with you just some of the “evidence” we have gathered. This evidence is comprised of; government press releases, published media, social media accounts, and official correspondence received by Pat from her former employer who remains her healthcare provider, Kaiser Permanente.
Government Press Releases – CA AG Rob Bonta:
“The settlement also includes injunctive relief terms to help prevent this type of crisis from reoccurring. It will result in court orders requiring Cardinal, McKesson, and AmerisourceBergen, for a period of 10 years, to:
- Establish a centralized independent clearinghouse to provide all three distributors and state regulators with aggregated data and analytics about where drugs are going and how often, eliminating blind spots in the current systems used by distributors;
- Use data-driven systems to detect suspicious opioid orders from customer pharmacies;
- Terminate customer pharmacies’ ability to receive shipments, and report those companies to state regulators when they show certain signs of drug diversion;
- Report and prohibit shipping of suspicious opioid orders;
- Prohibit sales staff from influencing decisions related to identifying suspicious opioid orders; and
- Require senior corporate officials to engage in regular oversight of anti-diversion efforts.”
“This settlement will not only bring resources to our state, cities, and counties to help fund treatment and recovery, it will help prevent these companies from ever again engaging in the improper business practices that led to the ongoing crisis.”
Published media:
“Kirkpatrick said chain pharmacies stopped filling his patients prescriptions after a 2021 National Opioid Settlement. The settlement created strict limits of potentially addictive drugs, drug distributors supplied to pharmacies. Cardinal Health, a drug distributor, supplies pharmacies throughout Longview. … The settlement also created red flag thresholds for opioid prescriptions, Kirkpatrick said. Flags may be raised if pharmacies prescribe an inordinate share of opioids in their areas, or if doctors prescribe an inordinate amount. … But when many pharmacies stop filling opioid prescriptions, and some doctors stop prescribing the pain-medication, it can create a cascading effect of flags being triggered. This could occur as individual doctors prescribe, and pharmacies fill, a high portion of opioids in a given area, Kirkpatrick said. … Recently, Kirkpatrick said Cardinal Health, the drug distributor for Longview, told independent pharmacies not to honor Kirkpatrick’s opioid prescriptions. … A local pharmacist told us he agrees with Kirkpatrick’s concerns.” (my emphasis added) Longview patients unable to get refills for opiod prescriptions | kgw.com 3-5-24
“Amid a nationwide opioid crisis, some Hawaii pharmacies are increasingly cautious about how they dispense pain medication … The prescription pills problem is a nationwide issue and follows years of litigation over America’s opioid crisis. A 2021 national settlement created strict limits on who (how) the drugs are distributed.” (my emphasis added) Amid opioid crisis, chronic pain patients are struggling to fill their needed prescriptions (hawaiinewsnow.com) 3-14-24
“A few months ago, I got a text from one of the smartest pharmacists I know, who owns an independent pharmacy that has been in his family for three generations. He knows the business, cold, and consults for many other independent pharmacies. He told me, somewhat panicked and despondent, that big distributors are no longer allowing most pharmacies access to controlled substances, which is between 10-15% of the business. His pharmacy was dispensing various controlled substances, as every pharmacist does, and one day was simply cut off from this class of drugs by the wholesaler/distributor he buys medicine from, which is a firm named Cardinal Health. … the wholesalers and their secret rules. A pharmacist can’t call up and find out whether they are violating them.” The Monopolies Behind the Adderall Shortage (thebignewsletter.com) 3-23-23
Distributor & Pharmacy:
“…, retail pharmacy customers can expect that the pharmacy questionnaires, customer interviews, and due diligence requirements currently in use by one or more distributors will also change as the distributors adapt to the specific injunctive relief terms regarding customer diligence. Lastly, under the injunctive relief terms, retail pharmacy customers that are terminated from, or declined to be onboarded for, controlled substances ordering due to concerns regarding the customer’s ability to provide effective controls against the potential diversion of controlled substances will be reported to the state attorney general’s office for their location.” Proposed Opioid Litigation Comprehensive Settlement and Injunctive Relief Terms – February 25, 2022 (cardinalhealth.com)
“Below is a 23 item questionnaire apparently given to a pt to have their prescriber to fill out. Apparently so that Walgreens could decide if they were going to fill this particular prescriber’s Rxs going forward. I have no idea which Walgreens store it came from since there is no address on the form. So it must be a generic form that is used for all the 9,000 odd Walgreen USA pharmacies.” (A “screenshot” of the actual questionnaire is available at this link.) DEA & surrogates are trying to throttle the availability of controlled meds to pts PHARMACIST STEVE 5-1-23
Selected Social Media: (w/emphasis added)
“My pharmacy is unable to get my pain medication in stock. One employee also mentioned that they try, but the DEA is getting even more difficult. Such as requiring they vet the doctors and pain patients.“ 3-8-24
“The pharmacy today informed me that they will not fill my pain meds. The pharmacist said I have to go to pain management.” 2-7-24
“It’s not even the doctor’s, the government has stopped the manufacturers from distributing to pharmacies! Including hospital pharmacies.” 3-10-24
Kaiser/The Permanente Medical Group (TPMG):
“These protocols in part are in place and rigid due to pressure pharmaceutical companies not providing meds to pharmacies who are not complying with these guidelines. So it is not just federal regulations that are dictating decisions made by TPMG.” (Response to a grievance by the Assistant Chief of Adult and Family Medicine, Kaiser Vacaville 1-14-23)
“Kaiser Permanente prescribers and pharmacists are reviewing opioid treatment plans and prescriptions in response to new pain management recommendations issued by CDC, and to legal settlement between wholesale drug distributors and the government.” … Because of nationwide changes in supplies, prescribing and dispensing controlled substances affecting both KP and non-KP pharmacies, some pharmacies may run out of certain medications.” (A letter to all Northern California chronic pain patients from NCAL Pharmacy Operations and Services 1-23-23)
IN CONCLUSION
If you are still reading this, and we have piqued your interest sufficiently, we suggest you read the aforementioned “Injunctive Relief/Exhibit P” for yourself. You will find it at page 478 of the 571-page settlement at this link: Final-Distributor-Settlement-Agreement-3.25.22-Final.pdf . But, for those still not desiring to take on all 54 pages at this time, I have included some selected excerpts compiled as a “Readers Digest” version of the injunction. Enjoy!!!
Selected Excerpts from the Injunctive Relief/Exhibit-P
INTRODUCTION
- Within ninety (90) days of the Effective Date unless otherwise set forth herein, each Injunctive Relief Distributor shall implement the injunctive relief terms set forth in Sections II through XIX (the “Injunctive Relief Terms”) in its Controlled Substance Monitoring Program (“CSMP”).
- The Effective Date of these Injunctive Relief Terms shall be defined by Section I.P of the Settlement Agreement, dated as of July 21, 2021, which incorporates these Injunctive Relief Terms as Exhibit P.
Section III. DEFINITIONS
- “Dispensing Data.” Includes, unless altered by the Clearinghouse Advisory Panel: (i) unique patient IDs; (ii) patient zip codes; (iii) the dates prescriptions were dispensed; (iv) the NDC numbers of the drugs dispensed; (v) the quantities of drugs dispensed; (vi) the day’s supply of the drugs dispensed; (vii) the methods of payment for the drugs dispensed; (viii) the prescribers’ names; (ix) the prescribers’ NPI or DEA numbers; and (x) the prescribers’ zip codes or addresses.
- “Highly Diverted Controlled Substances.” Includes: (i) oxycodone; (ii) hydrocodone; (iii) hydromorphone; (iv) tramadol; (v) oxymorphone; (vi) morphine; (vii) methadone; (viii) carisoprodol; (ix) alprazolam; and (x) fentanyl.
- “Pharmacy Customer Data.” Aggregated and/or non-aggregated data provided by the Customer for a 90-day period.
- To the extent feasible based on the functionality of a Customer’s pharmacy management system, Pharmacy Customer Data shall contain (or, in the case of non-aggregated data, shall be sufficient to determine) the following:
- a) A list of the total number of prescriptions and dosage units for each NDC for all Controlled Substances and non-Controlled Substances;
- b) A list of the top five prescribers of each Highly Diverted Controlled Substance by dosage volume and the top ten prescribers of all Highly Diverted Controlled Substances combined by dosage volume. For each prescriber, the data shall include the following information:
(1) Number of prescriptions and doses prescribed for each Highly Diverted Controlled Substance NDC;
(2) Number of prescriptions for each unique dosage amount (number of pills per prescription) for each Highly Diverted Controlled Substance NDC;
(3) Prescriber name, DEA registration number, and address; and
(4) Medical practice/specialties, if available;
- c) Information on whether the method of payment was cash for (a) Controlled Substances, and (b) non-Controlled Substances; and
- d) Information on top ten patient residential areas by five-digit ZIP code prefix for filled Highly Diverted Controlled Substances by dosage volume, including number of prescriptions and doses for each Highly Diverted Controlled Substance NDC.
- “Suspicious Orders.” As defined under federal law and regulation and the laws and regulations of the Settling States that incorporate the federal Controlled Substances Act. Suspicious Orders currently include, but are not limited to, orders of unusual size, orders deviating substantially from a normal pattern, and orders of unusual frequency.
- “Threshold.” The total volume of a particular drug family, DEA base code, or a particular formulation of a Controlled Substance that an Injunctive Relief Distributor shall allow a Customer to purchase in any particular period.
- “Top Prescriber.” A prescriber who, for a Customer, is either (i) among the top five (5) prescribers of each Highly Diverted Controlled Substance or (ii) among the top ten (10) prescribers of Highly Diverted Controlled Substances combined, as determined from the most recent Pharmacy Customer Data for that Customer.
Section VIII. Red Flags
- For purposes of the Injunctive Relief Terms, “Red Flags” are defined as follows:
- Ordering ratio of Highly Diverted Controlled Substances to nonControlled Substances: Analyze the ratio of the order volume of all Highly Diverted Controlled Substances to the order volume of all nonControlled Substances to identify Customers with significant rates of ordering Highly Diverted Controlled Substances.
- Ordering ratio of Highly Diverted Controlled Substance base codes or drug families to non-Controlled Substances: Analyze the ratio of the order volume of each Highly Diverted Controlled Substance base code or drug family to the total order volume of all non-Controlled Substances to identify Customers with significant rates of ordering each Highly Diverted Controlled Substance base code or drug family.
- Excessive ordering growth of Controlled Substances: Analyze significant increases in the ordering volume of Controlled Substances using criteria to identify customers that exhibit percentage growth of Controlled Substances substantially in excess of the percentage growth of non-Controlled Substances.
- Unusual formulation ordering: Analyze ordering of Highly Diverted Controlled Substances to identify customers with significant ordering of high-risk formulations. High-risk formulations include, but are not limited to, 10mg hydrocodone, 8mg hydromorphone, 2mg alprazolam, single ingredient buprenorphine (i.e., buprenorphine without naloxone), and highly-abused formulations of oxycodone. On an annual basis (or as otherwise necessary), high-risk formulations of Highly Diverted Controlled Substances may be added, removed, or revised based on the Injunctive Relief Distributors’ assessment and regulatory guidance.
- Out-of-area patients: Analyze Pharmacy Customer Data or Dispensing Data to assess volume of prescriptions for Highly Diverted Controlled Substances for out-of-area patients (based on number of miles traveled between a patient’s zip code and the pharmacy location, depending on the geographic area of interest) taking into consideration the percentage of out-of-area patients for non-Controlled Substances.
- Cash prescriptions: Analyze Pharmacy Customer Data or Dispensing Data to assess percentage of cash payments for purchases of Controlled Substances taking into consideration the percentage of cash payments for purchases of non-Controlled Substances.
- Prescriber activity of Customers: Analyze Pharmacy Customer Data or Dispensing Data to identify Customers that are dispensing Highly Diverted Controlled Substance prescriptions for Top Prescribers as follows:
- a) Top Prescribers representing a significant volume of dispensing where the prescriber’s practice location is in excess of 50 miles from the pharmacy (“out-of-area”), relative to the percentage of out-of-area prescriptions for non-Controlled Substances.
- b) Top Prescribers representing prescriptions for the same Highly Diverted Controlled Substances in the same quantities and dosage forms indicative of pattern prescribing (e.g., a prescriber providing many patients with the same high-dose, high-quantity supply of 30mg oxycodone HCL prescription without attention to the varying medical needs of the prescriber’s patient population).
- c) Top Prescribers where the top five (5) or fewer prescribers represent more than fifty percent (50%) of total prescriptions for Highly Diverted Controlled Substances during a specified period.
- & 9. Intentionally Omitted
- Customer termination data: Review information from the Injunctive Relief Distributor’s due diligence files and, when operable, from the Clearinghouse, subject to Section VIII.F, regarding Customers that have been terminated from ordering Controlled Substances by another distributor due to concerns regarding Controlled Substances.
- ONBOARDING
- Obtain a “Pharmacy Questionnaire” completed by the owner and/or pharmacist-in-charge of the potential Customer. The Pharmacy Questionnaire shall require going-concern potential Customers to list their top ten (10) prescribers for Highly Diverted Controlled Substances combined, along with the prescriber’s specialty, unless the Injunctive Relief Distributor is able to obtain this data otherwise. The Pharmacy Questionnaire shall also require disclosure of the identity of all other distributors that serve the potential Customer, and whether the potential Customer has been terminated or suspended from ordering Controlled Substances by another distributor and the reason for any termination or suspension. The Pharmacy Questionnaire shall request information that would allow the Injunctive Relief Distributor to identify Red Flags, …
ONGOING DUE DILIGENCE
- On an annual basis, each Injunctive Relief Distributor shall obtain updated pharmacy questionnaires from five hundred (500) Customers to include the following:
- The top 250 Customers by combined volume of Highly Diverted Controlled Substances purchased from the Injunctive Relief Distributor measured as of the end of the relevant calendar year;
- Additional Customers selected as a representative sample of various geographic regions, customer types (Independent Retail Pharmacy Customers and Chain Customers), and distribution centers.
XII. THRESHOLDS
- Each Injunctive Relief Distributor shall use Thresholds to identify potentially Suspicious Orders of Controlled Substances from Customers.
- Each Injunctive Relief Distributor’s CSMP department shall be responsible for the oversight of the process for establishing and modifying Thresholds. The sales departments of the Injunctive Relief Distributors shall not have the authority to establish or adjust Thresholds for any Customer or participate in any decisions regarding establishment or adjustment of Thresholds.
- Injunctive Relief Distributors shall not provide Customers specific information about their Thresholds or how their Thresholds are calculated.
- Threshold Setting
- a) Injunctive Relief Distributors shall primarily use model-based thresholds. For certain circumstances, Injunctive Relief Distributors may apply a non-model threshold based on documented customer diligence and analysis.
- b) Each Injunctive Relief Distributor shall include in its Annual Threshold Analysis and Assessment Report (as required by Section XVIII.F.3.c) to the Monitor summary statistics regarding the use of non-model thresholds and such information shall be considered by the Monitor as part of its Threshold Setting Process Review in the annual Audit Report.
- c) For the purposes of establishing and maintaining Thresholds, each Injunctive Relief Distributor shall take into account the Controlled Substances diversion risk of each drug base code. The diversion risk of each base code should be defined and reassessed annually by the Injunctive Relief Distributor’s CSMP Committee and reviewed by the Monitor.
- d) Each Injunctive Relief Distributor shall establish Thresholds for new Customers prior to supplying those Customers with Controlled Substances and shall continue to have Thresholds in place at all times for each Customer to which it supplies Controlled Substances.
- e) When ordering volume from other distributors becomes readily available from the Clearinghouse, an Injunctive Relief Distributor shall consider including such information as soon as reasonably practicable in establishing and maintaining Thresholds.
- f) Each Injunctive Relief Distributor shall incorporate the following guiding principles in establishing and maintaining Customer Thresholds, except when inapplicable to non-model Thresholds:
(1) Thresholds shall take into account the number of nonControlled Substance dosage units distributed to, dispensed and/or number of prescriptions dispensed by the Customer to assist with the determination of Customer size. As a general matter, smaller customers should have lower Thresholds than larger customers.
(2) For the purposes of establishing and maintaining Thresholds, Injunctive Relief Distributors shall use statistical models that are appropriate to the underlying data.
(3) For the purposes of establishing and maintaining Thresholds, Injunctive Relief Distributors shall take into account a Customer’s ordering and/or dispensing history for a specified period of time.
(4) For the purposes of establishing and maintaining Thresholds, Injunctive Relief Distributors shall take into account the ordering history of Customers within similar geographic regions, or, where appropriate for Chain Customers, ordering history within the chain.
(5) If appropriate, Thresholds may take into account the characteristics of Customers with similar business models. (a) A Customer’s statement that it employs a particular business model must be verified, to the extent practicable, before that business model is taken into account in establishing and maintaining a Customer’s Threshold.
- Threshold Auditing
- a) The Injunctive Relief Distributors shall review their respective Customer Thresholds at least on an annual basis and modify them where appropriate.
- b) Each Injunctive Relief Distributor’s CSMP department shall annually evaluate its Threshold setting methodology and processes and its CSMP personnel’s performance in adhering to those policies.
- Threshold Changes
- a) An Injunctive Relief Distributor may increase or decrease a Customer Threshold as set forth in its CSMP policies and procedures, subject to Sections XII.C.3.b through XII.C.3.e.
- b) Prior to approving any Threshold change request by a Customer, each Injunctive Relief Distributor shall conduct due diligence to determine whether an increase to the Threshold is warranted. This due diligence shall include obtaining from the Customer the basis for the Threshold change request, obtaining and reviewing Dispensing Data and/or Pharmacy Customer Data for the previous three (3) months for due diligence purposes, and, as needed, conducting an on-site visit to the Customer. This Threshold change request diligence shall be conducted by the Injunctive Relief Distributor’s CSMP personnel.
- c) No Injunctive Relief Distributor shall proactively contact a Customer to suggest that the Customer request an increase to any of its Thresholds, to inform the Customer that its Orders-to-date are approaching its Thresholds or to recommend to the Customer the amount of a requested Threshold increase. It shall not be a violation of this paragraph to provide Chain Customer headquarters reporting on one or more individual Chain Customer pharmacy location(s) to support the anti-diversion efforts of the Chain Customer’s headquarters staff, and it shall not be a violation of this paragraph for the Injunctive Relief Distributor’s CSMP personnel to contact Customers to seek to understand a Customer’s ordering patterns.
- d) An Injunctive Relief Distributor’s Chief Diversion Control Officer may approve criteria for potential adjustments to Customer Thresholds to account for circumstances where the Thresholds produced by the ordinary operation of the statistical models require modification. Such circumstances include adjustments to account for seasonal ordering of certain Controlled Substances that are based on documented diligence and analysis, adjustments made to permit ordering of certain Controlled Substances during a declared national or state emergency (e.g., COVID-19 pandemic), IT errors, and data anomalies causing results that are inconsistent with the design of the statistical models. Each Injunctive Relief Distributor shall include in its Annual Threshold Analysis and Assessment Report (as required by Section XVIII.F.3.c) to the Monitor information regarding the use of this paragraph and such information shall be considered by the Monitor as part of its Threshold Setting Process Review in the annual Audit Report.
- e) Any decision to raise a Customer’s Threshold in response to a request by a Customer to adjust its Threshold must be documented in a writing and state the reason(s) for the change. The decision must be consistent with the Injunctive Relief Distributor’s CSMP and documented appropriately.
Section XVII. CLEARINGHOUSE
- Phase 1 of the Clearinghouse: Data Collection, Initial Analytics and Reporting
- Additional Reports and Analytics
- f) The Clearinghouse shall also prepare reports and analyses for the Settling States and Injunctive Relief Distributors identifying prescribers whose prescribing behavior suggests they may not be engaged in the legitimate practice of medicine. Such reports and analysis shall … seek to identify and evaluate:
(1) Prescribers who routinely prescribe large volumes of Highly Diverted Controlled Substances relative to other prescribers with similar specialties, including health care professionals who prescribe a large number of prescriptions for high dosage amounts of Highly Diverted Controlled Substances;
(2) Prescribers whose prescriptions for Highly Diverted Controlled Substances are routinely and disproportionately filled in a geographic area that is unusual based on the prescriber’s location; and
(3) Prescribers who routinely prescribe out-of-specialty or out of-practice area without legitimate reason.














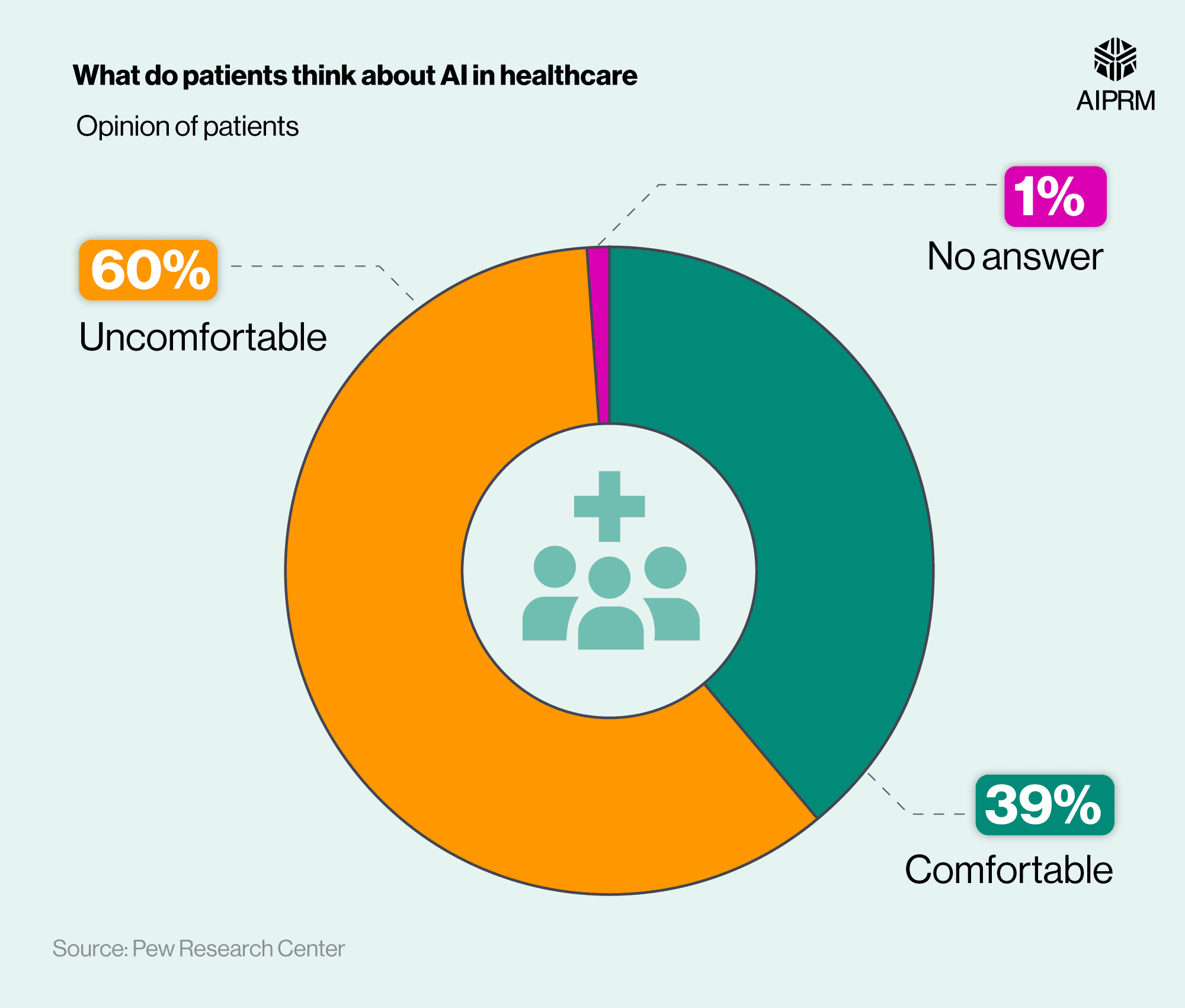

It’s simply a war on controlled medication. Thanks for all the work you and Pat have done in this.